By Aaron Krol
June 5, 2015 | In
Stephen Elledge’s lab at the Harvard Longwood Campus in Boston, a unique
collection of viruses lies dormant in a refrigerated slurry. They are
bacteriophages ― viruses that prey on bacteria ― and harmless to us, but each
of the nearly 100,000 varieties in the mixture does share something in common
with a virus that infects humans. Specifically, each one has been engineered to
create a single peptide, a fragment of a protein, that would normally be found
in a human pathogen ― from the rhinoviruses behind the common cold, to notorious
killers like HIV and the West Nile virus. The phages wear these peptides on
their outer coats like identifying tags.
Together, the 100,000 tags in this viral goulash span every
protein of every human-infecting virus in the vast UniProt database, where scientists
around the world document protein structures. In effect, the slurry is a living
library of viral peptides, which Elledge and his colleagues at the Howard
Hughes Medical Institute of Brigham & Women’s Hospital have created to study the human virome, the vast and
mysterious set of viruses that live in our bodies.
In a paper published this week
in Science, Elledge and his
coauthors, led by graduate students Tomasz Kula and George Xu, unveiled a new
method that uses this phage mixture to test blood samples for over 200 species
and 1,000 strains of virus at a time. The team speculates that their technique,
named VirScan (VEERscan), could one
day become a near-universal test for viral infections using just one drop of a
patient’s blood, replacing one-off tests for specific types of virus.
“You’re looking across all viruses at once, without having
to suspect ahead of time that maybe there’s a particular infection,” says Kula.
“This opens up a lot of questions that simply couldn’t be asked before, because
it wouldn’t be practical to look at every single virus individually.”
Is There Antibody in There?
The virome doesn’t get as much love as its charismatic older
brother, the microbiome. Studies of the bacteria that live inside us have
caught the public imagination, showing that we contain a teeming diversity of
critters whose populations affect everything from our diets to our immune
systems. Thanks to cheap DNA sequencing, you can send samples of your
microbiome to a lab and have a quick census taken; services like American Gut
will even give you a colorful chart showing you which bacteria have been found
and in what numbers. (Strictly the virome is part of the microbiome, which
includes all the viruses, protozoa, and fungi living in one environment ― but
bacteria are the stars of the show.)
Scientists can and do use DNA sequencing to study viruses
too, but for several reasons, viral DNA is hard to work with. “Viruses don’t
have a single gene that can be assayed like bacteria do,” says Kristine Wylie,
a member of the McDonnell Genome Institute of Washington University who studies
the human virome. With bacteria, researchers often start genetic tests by amplifying a
gene called 16S rRNA, which all bacteria share some version of, but viruses
don’t have one target that can be amplified. “Viruses have DNA genomes, RNA
genomes, single-stranded, double-stranded ― it’s a very complex set of genomes
that are all lumped together.”
Without a shortcut like 16S rRNA, virome studies have to
comb through a lot of genetic material, most of which belongs to the viruses’
human hosts or other microorganisms. This signal-to-noise problem is made worse
by the fact that viruses can be very shy. You might think a blood sample would
turn up any viruses circulating in the body, but at different points in their
lifecycles, viruses might be hiding quietly in the liver, or be present at such
low levels that sequencing won’t pick them up. “One of the challenges is that
you’re only going to capture the viruses that are circulating in the blood in
large enough numbers,” says Kula.
This is the kind of problem the Elledge lab specializes in
solving. Kula and his colleagues work on technologies that can run large
volumes of complex lab procedures at once, from cloning DNA fragments to designing
“promoters” that enhance the activity of specific genes. With VirScan, the team
has taken this high-throughput approach to testing the human virome. Their
solution piggybacks on the human body’s own system for recognizing viruses:
antibodies, the proteins our immune cells produce to bind with the “epitopes” on
viruses’ outer coats.
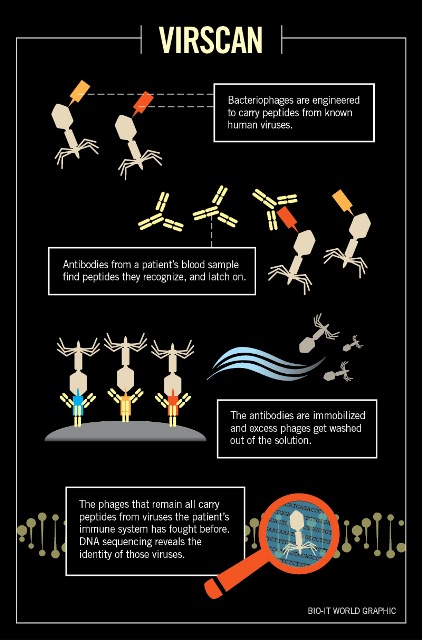
Unlike viruses, antibodies always circulate freely through
the blood, making them easy to scoop up in blood samples. Tests used in
hospitals to diagnose viral infections often look for the antibodies the immune
system creates in response to those infections. These tests, however, are
highly targeted: one test for one virus.
VirScan, on the other hand, can look at all the antibodies in a patient’s blood, by translating them into
the language of DNA. The translators are those 100,000 varieties of bacteriophage
with their special peptides.
It’s a simple process: the scientists mix a batch of phages with
a patient’s blood sample, and let the circulating antibodies in the blood do
their work, latching onto any phages whose epitopes they recognize. Once the
antibodies have had time to find their matching epitopes, the scientists pull them
out with magnetic beads and wash away any phages that haven’t been hooked.
Finally, DNA sequencing reveals which epitopes are still in
the sample, captured by the patient’s own antibodies. Since these epitopes come
from specific viruses documented in UniProt, each one can be matched to a viral
infection that the patient’s body is fighting or has fought in the past. In
this way, VirScan reveals much more than direct sequencing of a blood sample ―
including shy viruses or even remnants of infections from years before.
“To me, the big thing that is exciting about this is the
ability to look broadly,” says Wylie, who was not involved in the VirScan
research. “That’s just not something that we’ve been able to do with antibodies
before. A lot of work went into this, trying to be as comprehensive as they
could.”
Stand Up and Be Counted
It’s a little hard to tell how accurate VirScan is. So far,
the team at the Howard Hughes Medical Institute has run VirScan on nearly 600
patients, including groups from the U.S., Peru, South Africa, and Thailand ―
but because the technique looks for over 200 species of virus, the only way to
check every result would be to run 200 targeted tests on each patient.
Early analysis, however, looks promising. A good reality
check is simply to ask whether VirScan’s results are plausible: if the test
reported that only 5% of patients had influenza antibodies, or that 90% had
antibodies to the ebolavirus, that would be a red flag.
From this perspective, the results seem good. Very common
infections, like rhinoviruses and herpesviruses, come up regularly in VirScan,
while more exotic viruses are found rarely if at all. “Sure enough, for many
common viral infections we’re detecting them at pretty high levels,” says Kula.
“And for some viruses like CMV [one of the herpesviruses], which is known to
infect about half the population, that is in fact what we saw.”
In a few cases, the team was able to get much more exact
measures of accuracy, by recruiting patients who had already been tested for
specific viruses ― including HIV and the hepatitis C virus. With these
patients, VirScan agreed with other forms of testing more than 90% of the time,
a very good record for a brand-new technology. Importantly, in 97 tests VirScan
gave only one false positive result, claiming to find hepatitis C in a patient
who reported being hepatitis-negative. Even in that case, the team believes
their method may have picked up a past infection the patient was unaware of.
Nonetheless, there are a few results that don’t square with
what we know about the human virome. For example, if VirScan were perfectly accurate,
it should have detected poliovirus in almost every patient ― not because polio
is a common illness, but because most people around the world are vaccinated
against it, producing polio antibodies. Instead, poliovirus showed up in barely
a third of samples. Similarly, antibodies to the chickenpox virus were found in
less than a quarter of patients tested.
“What we pick up is just a snapshot of what antibodies you
have in your blood at the time when we draw it,” says Kula. That makes VirScan
sensitive to differences in how the immune system reacts to different viruses.
Small viruses with few epitopes can sneak by undetected; closely related strains
of virus can get confused; and infections from many years earlier can fade
below VirScan’s level of sensitivity, as the antibodies gradually disappear.
“Over time, you do maintain antibodies from infections in the past, but they do
wane,” Kula says.
“With any technology, particularly one that’s broad, it’s
going to work better for some things than others,” says Wylie. “[VirScan] is
broad, and from what they’ve shown it works well, but that doesn’t mean it’s
going to work perfectly for every virus… There’s always going to be limits of
detection.”
When it comes to scanning the entire virome, VirScan is best
thought of as a complement to ordinary sequencing studies. VirScan can pick up
past infections; sequencing can tell you which viruses are present in the
moment. VirScan can look quickly across all known human viruses; results from
sequencing might take longer to interpret, but they can uncover viruses no one
has seen before.
“I think there would be lots of applications of this
technology to go along with other research,” Wylie adds. “I’m pretty excited to
see how people might use it.”
Very Special Epitopes
Maybe the most intriguing thing about VirScan is what it can
tell us about our own immune systems.
Unlike past antibody tests, VirScan isn’t limited to a few
viral epitopes that are known to trigger an immune response. The peptide
library carried by the Elledge lab’s bacteriophages covers a huge variety of
viral proteins. Looking at those peptides reveals not only which viruses a patient has fought, but also
which specific peptides the immune system chose as its targets.
Even in their first round of 600 tests, Elledge’s team sees surprising
patterns in the results. Presented with a wealth of epitopes from each viral
species, our immune systems tend to choose from just a few ― often clustered
close together in the viral genome.
“A lot of people who have been infected by a virus will
actually generate antibodies against the same exact region,” says Kula. “This
definitely surprised us… Why there is this similarity could be very
informative.” In the paper, Kula and his colleagues suggest that some epitopes
could be more exposed on viruses’ coats than others, or that our immune cells
might create antibodies in non-random ways that bias them to certain peptide
sequences. At this stage, we really don’t know.
What’s clear is that VirScan could be brought into a number
of research areas where virome-wide sequencing, or targeted viral tests, have
not given us the full picture. Wylie, for instance, is curious what antibodies
would be found in a large study of children, a major focus of her own research
on the virome. “You’re actually developing your immune repertoire at that
time,” she says. “It would be interesting to see that history of what children
had been exposed to, and what might be missing from the common pathogens in a
child… We have to realize we’re a bit changed after these exposures, and this
is one method of looking broadly at that.”
Kula, meanwhile, hopes that VirScan can shine a light on mysterious
diseases like chronic fatigue syndrome and Kawasaki disease. Some experts
suspect these illnesses are caused or exacerbated by viruses, but those
suspicions are hard to confirm when no one knows which virus to look for. With
VirScan, this is no obstacle.
Not that every VirScan study needs to be so exotic. “Understanding
what viruses generally healthy people are carrying around is important,” says
Wylie. “And understanding what viruses healthy people are carrying that could
potentially be pathogens, should they become immunocompromised or have some
other issue that causes the virus to reactivate ― all of those things are
important.”
Like the microbiome, our relationship with the virome is
lifelong but little understood. Until they make us sick, our viruses are
invisible and seem inactive; but their battles with our immune systems, and the
ripple effects on new infections and other microorganisms, don’t fully fade
away with our fevers.
Instead, every virus we meet leaves its permanent mark ― if
only you know where to look.