Contributed Commentary By Matthew Michela, President and CEO, Life Image and Dan Housman, Co-founder and CTO, Graticule
February 20, 2020 | Radiology is increasingly the key to synthesizing real world evidence with sufficient quality to shape drug performance. As a diagnostic toolset, imaging is one of the oldest and most widely used and clearly interpreted methods for measurement of outcomes. It is regularly used to measure disease progression and to determine the effectiveness of medical interventions. For example, in neurology, imaging is used to determine progression in Multiple Sclerosis by establishing plaque accumulation over time. In oncology, imaging is used to measure solid tumor size and burden in response to or preparation for therapy. Imaging is also a common mechanism for early identification of disease that can be automated or extended through artificial intelligence (AI). This is one reason the industry has seen a fair amount of development activity in the use of AI in mammography for breast cancer and chest CTs to identify lung cancer, as these are two high-volume screening methods for catching cancer at a point early enough to successfully treat it.
With the advent of digitization of medical records, Real World Data (RWD) became a critical part of drug development and commercialization. While medical insurance claims were historically the primary source of information for analyzing treatment patterns and economic outcome, they lack the clinical details unrelated to the quantification of payment activity, which are needed to establish regulatory-grade medical evidence. The 21st Century Cures Act includes provisions for approving new indications for existing approved drugs based on RWD. But the burden of proof for regulatory submissions requires regulatory-grade data at a level similar to clinical trials. New drugs such as Amgen’s Blicyto and Roche’s Alecensa have successfully used synthetic control arms to obtain approval without needing a placebo, but these synthetic approaches require regulatory-grade data that can be compared with validated information from studies. In addition to approvals for drugs, biomarkers are now increasingly developed through AI training, and imaging can help identify patients through complex unstructured data. Biomarkers also have similar requirements for regulatory approvals to translate their use into clinical practice.
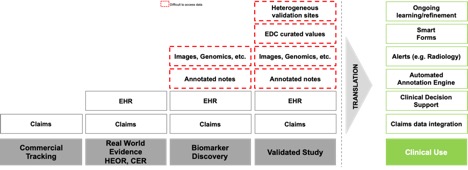
Advanced use cases such as biomarker discovery and validated studies require advanced data sets. Imaging and radiology notes have become critical in these scenarios. For example, in a synthetic control arm for a clinical trial, the patient will have a set of progression markers that are being evaluated relative to the treatment arm. However, because the data was originally read from an image for the purpose of care, the prudent approach is to obtain the original images and use them to create an independent read with the same rigor and content as the information captured within the clinical trial intervention population. Even if the radiology study is not read a second time, the original study likely needs to be available for any questions that may arise from a regulatory review regarding the validity of comparison between the two groups.
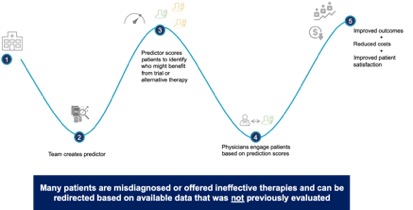
Additionally, digital biomarkers can be generated from imaging data to help identify patients for either clinical trial recruitment or treatment. The following use case illustrates how this can be done. A drug company has a product that can treat the rare disease Ankylosing Spondylitis (AS). The disorder is under-diagnosed because most patients complain of joint and back pain rather than of the underlying AS autoimmune disorder. By acquiring a training set of images for patients with and without AS, the drug company can build a tool to classify patients so that AS risk can be scored in all images within a health system taken for orthopedic pain. Once the algorithm is proven to have a strong enough predictive power, it can be implemented in a health system to identify patients as they are scanned or by running against the historical imaging studies for a population and prospectively as new patients are given MRIs. Patients who are likely affected can be tested, in this example, with a low-cost lab test to prove they have the disease. Positive patients can be either recruited into a clinical trial or prescribed available therapies. This process is already common in cancer detection in radiology reads to produce incidentalomas (cancer cases identified incidentally during imaging unrelated to a cancer screening). Using real world imaging data complimented by other structured and unstructured patient data will add many possibilities for new incidental findings that lead to treatment of otherwise undetected disease.
Multiple AI companies have been building algorithms to improve the radiology study read process or to establish novel biomarkers. Several offer capabilities to increase reliability by providing automation to detect features in radiology images such as chest x-rays or mammograms. These algorithms are often built in narrow conditions with a single health system supplying data. In order to perform at a level required for approval as a medical device, the algorithms need to demonstrate that they can operate consistently with a range of equipment manufacturers and on a heterogenous population including, for example, multiple races, ethnicities, genders, and other demographic characteristics which are appropriately collected from a variety of institutions. Real world imaging data provides the resource to validate these types of AI algorithms sufficient for FDA submission and approval as medical devices. Furthermore, by maintaining a subscription to updated imaging, the AI tools have the data necessary to continuously improve precision and avoid safety situations that arise from utilization of static or homogeneous data.
The value of imaging data is beginning to be recognized for its high clinical utility and value in the end-to-end evidence management process. The really hard part comes next—how to access, deidentify, aggregate, govern, normalize and combine these complex data sets with other forms of data so it can be put to work to improve research and help patients receive better care.
Matthew A. Michela, President and CEO of Life Image, has been a healthcare industry executive for 30 years, serving in leadership positions in both the payer and care management sectors. He joined Life Image in 2015 with the mission of democratizing data to create an interoperable healthcare ecosystem that creates a connected view of a patient’s journey. He can be reached at mmichela@lifeimage.com.
Dan Housman, Co-founder and CTO of Graticule, is a software start-up veteran with a demonstrated track record in health system analytics, real world evidence, and clinical trial optimization. He grew Recombinant Data (bootstrap) to $15M annual revenue prior to acquisition then grew ConvergeHEALTH by Deloitte for past 6 years. He can be reached at dhousman@graticule.life.